Physical and Chemical Changes
There are two types of changes, physical and chemical changes.
Physical Changes
- In a physical change, no new substances are formed.
- Changes in size, shape, state and colour of a substance are physical changes.
- In a physical change, the changes are temporary and can be easily reversed to form the original substance.
Example:
Melting of ice and freezing of water.

Examples of physical change:
- Making of ice-cream
- Boiling water into steam.
- Crystallization of sugar from its solution,
- Sublimation of camphor.
- Bending of glass tube by heating.
- Melting of wax.
- Evaporation of water.
- Glowing of an electric bulb
- Magnetising an iron bar by means of electricity.
- Dissolving sodium chloride in water.
Definition of Chemical Change
Chemical change is a permanent change which cannot be reversed and in which the chemical properties of a substance change with the change in its composition. It also alters the specific properties of a compound by bringing about a change in its molecular composition followed by a change in state,
All of us are familiar is the rusting of iron. If we leave a piece of iron in contact of air for some time, it is observe that the piece of iron acquires a film of brownish substance. This substance is called rust and the process is called rusting. Iron gates of parks or farmlands, iron benches kept in lawns and gardens, almost every article of iron, kept in the open gets rusted. At home you must have seen shovels and spades getting rusted when exposed to theatmosphere for some time.
Characteristics of Chemical Changes
- Heat, light or any other radiation may be given off or absorbed.
- Sound may be produced.
- A change in smell may take place or a new smell may be given off.
- A colour change may take place.
Examples:
i. Burning of Magnesium Ribbon
- Burning of a magnesium ribbon is a chemical change. When a magnesium ribbon is held over the flame of a burner, it burns with a dazzling white light to form magnesium oxide.
- Mg + O2 → MgO
- The magnesium oxide obtained on dissolving in water also forms a new substance, magnesium hydroxide which turns red litmus paper blue, indicating that it is basic in nature. Hence, the dissolving of magnesium oxide in water is a chemical change.
MgO + H2O → Mg(OH)2
ii. Reaction between Copper sulphate and Iron
- The reaction between copper sulphate and iron is a chemical change. When an iron object is placed in a copper sulphate solution, a chemical reaction takes place to give two new substances, iron sulphate and copper.
iii. Reaction Between Baking soda and Vinegar
The reaction between baking soda and vinegar is a chemical change. Baking soda is sodium bicarbonate and vinegar contains acetic acid. On mixing baking soda with vinegar, a chemical change takes place to form three new substances, sodium acetate, carbon dioxide and water.
Distinguish between Physical and Chemical Change
| Physical Change | Chemical Change |
| No new substance is formed. | New substance is formed. |
| Change is temporary. | Change is permanent. |
| It is easily reversible. | It is usually irreversible. |
Protective Shield of Ozone
- There is a layer of ozone gas high up in the atmosphere.
- The ozone layer protects us from the harmful radiations coming from the Sun.
- The ozone absorbs these harmful radiations and breaks down to form oxygen.
- This breaking down of ozone into oxygen is a chemical change.
Rusting of Iron
Iron objects, on being left in damp air (or water) for a considerable period of time get covered with a red flaky substance called rust. This is called rusting of iron.
Iron combines with oxygen in the air, in the presence of water (moisture), to form iron oxide. This hydrated iron (III) oxide is nothing but rust.

Conditions Necessary for Rusting
- Two reasons necessary for rusting are:
- Presence of oxygen (in the air)
- Presence of water or water vapour (moisture)
How to Prevent Rusting?
Methods to prevent rusting of iron: Painting or Greasing: Applying a coat of paint or grease to the surface of the iron object prevents the surface from coming in contact with the air and moisture and thus prevents rusting.
Galvanisation: The process of depositing a layer of zinc on iron is called galvanisation. A thin layer of zinc is deposited on the surface of the iron object and protects it from rusting.
Alloying: Stainless steel, an alloy is made by mixing iron with carbon and metals such as chromium, nickel and manganese, so that it does not rust.
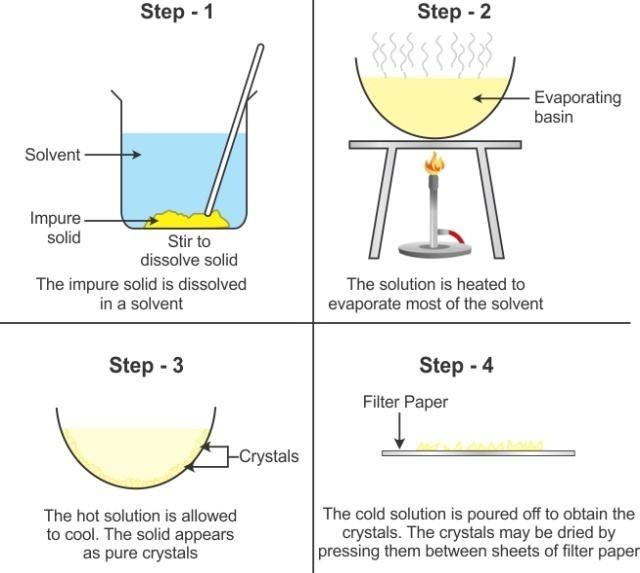
Crystallisation: The salt obtained from seawater by the process of evaporation is not pure and its crystals are small. Moreover, the shape of the crystals cannot be seen clearly.
However, large crystals of pure substances can be obtained from their solutions by the process of crystallisation.
The process of cooling a hot, concentrated solution of a substance to obtain crystals is called crystallisation.
About Energy Changes
All chemical reactions take place either by the absorption or the release of energy, generally in the form of heat energy.
Exothermic reactions:
These chemical reactions which proceed with the release of heat energy, are called exothermic reactions.
For example, When magnesium ribbon is heated from its tip in a Bunsen flame, it catches fire and burns with a dazzling white flame with release of heat and light energy. The product formed is magnesium oxide.

Process of Crystallisation
Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling.
The process is employed in the separation of pure common salt from the impure common salt obtained from sea. Similarly, it is used to obtain pure nitre from impure nitre (KNO3) pure copper sulphate (CuSO4), from its impure sample and pure alum (phitkari) from the impure alum.
- Separation of pure copper sulphate from impure sample:
10 g of impure sample of copper sulphate, containing sand particles or dirt particles an impurity is taken in a beaker. This sample is dissolved in minimum amount of water. The impurities are filtered and the clear copper sulphate solution is collected in a china dish. The china dish containing copper sulphate solution is placed over sand bath.
When sand is heated in an iron vessel by placing it over a tripod stand, this arrangement is called sand bath.
The solution is allowed to evaporate, so that more than half of the volume of water evaporates. The solution is now saturated at higher temperature. The china dish is removed from the sand bath and covered with filter paper. The copper sulphate solution in it is allowed to cool for 24 hours. After 24 hours blue crystals of copper sulphate are separated out. The crystals are filtered and dried in the folds of filter paper.
GALVANISATION
The process in which depositing a layer of zinc on iron occurs is known as galvanisation. The iron pipes we use in our homes to carry water are galvanised to prevent rusting. You know that ships are made of iron and a part of them remains under water. On the part above water also water drops keep clinging to the ship’s outer surface. Moreover, the water of the sea contains many salts. The salt water makes the process of rust formation faster. Therefore, ships suffer a lot of damage from rusting in spite of beingpainted. So much so, that a fraction of ship’s iron has to be replaced every year.
Corrosion of Aluminium:
Due to the formation of a dull layer of aluminium oxide when exposed to moist air, the aluminium metal loses its shine very soon after use. This aluminium oxide layer is very tough and prevents the metal underneath from further corrosion (because moist air is not able to pass through this aluminium oxide layer). This means sometimes corrosion is useful.
Corrosion of Copper:
When a copper object remains in damp air for a considerable time, then copper reacts slowly with carbon dioxide and water of air to form a green coating of basic copper carbonate [CuCO3.Cu(OH)2] on the surface of the object. Since copper metal is low in the reactivity series, the corrosion of copper metal is very, very slow.
Corrosion of Silver:
Silver is a highly unreactive metal, so it does not reacts with oxygen of air easily. But, air usually contains a little of sulphur compounds such as hydrogen sulphide gas (H2S), which reacts slowly
with silver to form a black coating of silver sulphide (Ag2S). Silver ornaments gradually turn black due to the formation of a thin silver sulphide layer on their surface and silver is said to be tarnished.
